More so than any other sectors, startups in the fields of medical and healthtech need professional validation from day one, in order to attract users and have any chance of succeeding.
For DyCare, a musculoskeletal rehabilitation digital solutions provider founded in Barcelona in 2015, validation came early: soon after the company started, the pharmaceutical giant Bayer selected it to join its incubation program. To date, DyCare has received more than €2m of funding from the public and private sectors.
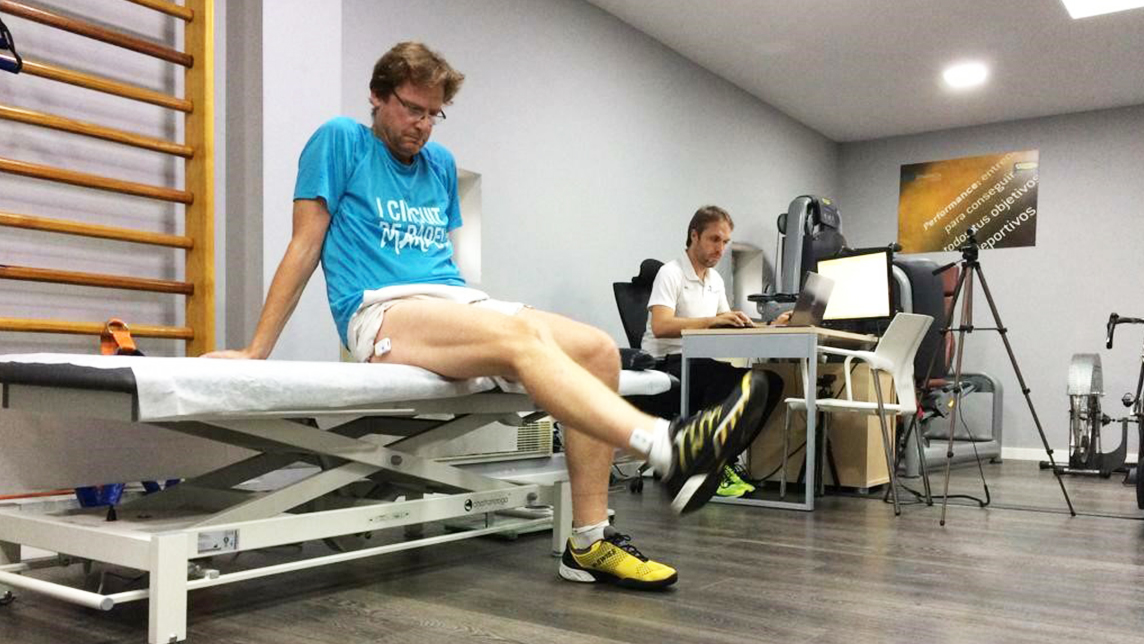
It currently offers two products for tracking the progress of musculoskeletal rehabilitation: Lynx and ReHub. Already available in some markets, Lynx consists of wearable sensors fixed to any joint, and connected via Bluetooth to a platform used by medical professionals for real-time evaluation of the patient's movements.
Its second product, ReHub, is a more scalable version due for commercial release in 2020. It uses the same core technology, but connects the GP, physiotherapist and patient via the cloud instead, while adding a patient exercise kit.
“Our platforms give us a snapshot in real time and we can see if there is any limitation of movement, analyze all the biomedical parameters and generate a report for the medical professionals allowing personalization of needs,” DyCare CEO and co-founder Silvia Raga told CompassList at the 2019 edition of 4YFN.
Having achieved European Union certification as medical devices, the platforms are now being commercialized across the EU, a market that has more than 15,000 musculoskeletal treatment centers and worth as much as €40bn, the company says.
“In Europe alone, there are more than 100m people with musculoskeletal problems and the majority are seeking rehabilitation,” Raga said.
An underserved sector
Raga has a background in healthcare management and holds a master's degree in Biomedical Engineering. She teamed up with Ricardo Jauregui, a medical doctor who is now the company's CTO and co-founder. At the time, both had needed physical rehabilitation and found existing services to be lacking. Raga said the lack of personalized treatment and regular monitoring of progress made the pair realize the sector was ripe for technological disruption.
Launched in February 2017, the Lynx platform consists of wearable sensors which are adaptable to any joint. Each wearable is equipped with two or four sensors depending on whether a patient needs a simultaneous biomechanical comparison of a healthy and a pathological joint. The wearables communicate data via Bluetooth to a platform for medical professionals, and use ML to transform the data into precise biomechanical parameters, allowing for real-time evaluation of movements.
Lynx also provides 3D visualization of the patient's mobility and automatically generates a complete functional assessment report for inclusion in medical records. The cost to clinics is €3,500 per two-sensor unit and €4,500 for four-sensor units.
ReHub incorporates a cloud-based 5G application designed for collaborative use between the doctor, physiotherapist and the patient. It has been approved for use in the EU and Switzerland and is being readied for commercial release in 2020. Unlike Lynx, it will not be retailed as a single unit, but via a SaaS license charged according to the number of users.
In addition to Lynx's capabilities for joint analysis, ReHub includes a smart exercise kit that is individually programmed for the patient by the physiotherapist. The home exercise program has more than 150 possible exercises for different muscle regions which the physiotherapist can adapt remotely. The patient is guided through the exercises and receives real-time visual biofeedback on their dashboard. A system of alerts is used to monitor if the patient exercises incorrectly or experiences pain. Reports on the patient's progress and comparisons with previous sessions are automatically generated for the GP and the physiotherapist.
Backed by pharmas, doctors
DyCare was quick to attract big-name supporters. Soon after its founding, the startup won a spot in Bayer's six-month incubation program as part of the first Spanish chapter of its BayerGrants4Apps initiative. Under the program, DyCare received expert mentoring and assessment, exposure to potential investors, and a physical base from which to develop its Lynx platform.
The company received €40,000 in funding for young entrepreneurs from the European Union Agency for Network and Information Security (Enisa) and €50,000 through the EU's innovation program Horizon 2020. From there, it secured partnerships with medical institutions that gave it access to clinical trials, and larger tranches of funding which enabled the company to avoid an external funding round.
In 2016, the Spanish arm of Italian pharma Chiesi signed a partnership agreement with DyCare to trial Lynx in monitoring trembling experienced by patients who had recently undergone transplant surgery. The three-month trial was successful in quantifying the level of trembling in each patient and the effects of various drugs used to treat the problem.
The following year, Dycare began a year-long trial at the University Hospital of Zurich in Switzerland to use Lynx on hand surgery patients. The satisfactory results led to the company being granted a license to sell its product in the Alpine nation.
Dycare received more than €2m in funding in 2018, allowing it to complete the development of ReHub. This money comprised a further €1m in EU funding from the SME Instrument program and €1m from private investors, mostly Swiss doctors.
“The best thing about this funding, besides allowing us to accelerate our development, was securing the support of the European Commission for our project,” Raga said.
Eyes 2020 funding
The company has since signed a partnership agreement with the International Osteoarthritis Foundation to use Lynx to assess joint function pre and post medical intervention. Until now, such assessments have been conducted via patient surveys.
Lynx is also being trialed in Spanish and Italian public hospitals. In addition, DyCare has secured 30 private Spanish clients including insurers Activa Mutua and MAZ, medical rehabilitation centers Fisomèdic Grup and Punto Vital and athletic training organization the High-Performance Center (Centro de Alto Rendimiento).
DyCare's medical advisory board includes prominent doctors Alessandro Castagna, Professor of Orthopaedics at UNIMED University (Milan); Marco Conti, a renowned shoulder and elbow consultant in Italy and Spain; and Maurizio Calcagni, General Secretary of the Federation of the European Societies for Surgery of the Hand.
DyCare plans to launch a financing round in early 2020. Monies would go toward the commercial of launch of ReHub and gaining market traction. It also has plans to enter the markets of Holland, Italy, the UK and Germany. US market entry remains a long-term goal given the country's different certification of quality assurance required for medical devices.
DyCare expects to have revenues of €4m in three years, and will become “the reference rehabilitation system for musculoskeletal disorders," Raga said.